
Breath sampling and analysis for biomonitoring and early disease diagnosis
10 December, 2019
Benefits of breath sampling
Breath analysis by TD–GC–MS holds the promise of non-invasive, early detection for life-threatening conditions such as lung cancer, breast cancer, liver disease and inflammatory bowel disease, all of which have been found to influence the profile of volatile organic compounds (VOCs) in the breath.
Outputs of breath analysis reveal a complex and diverse composition. The value lies in finding those compounds (biomarkers) whose concentrations consistently show differences between the breath of patients and that of healthy controls (see Figure 1).
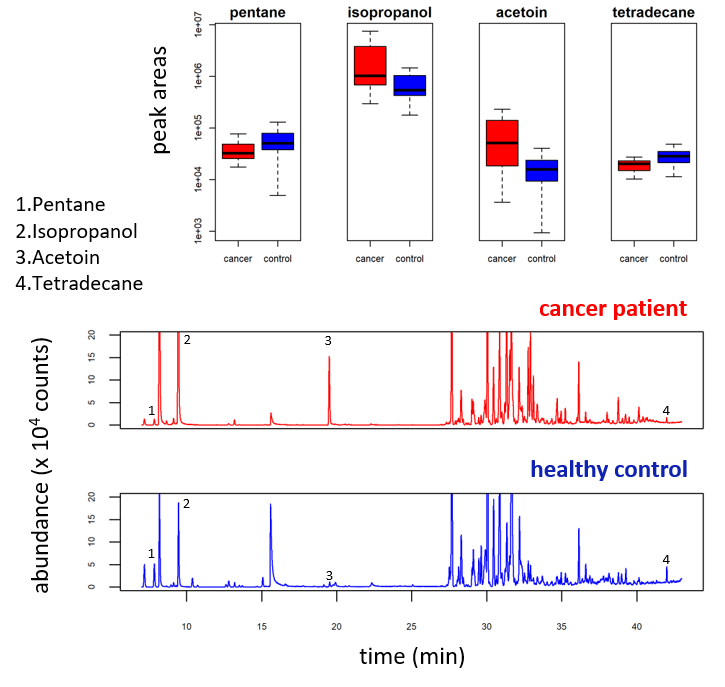
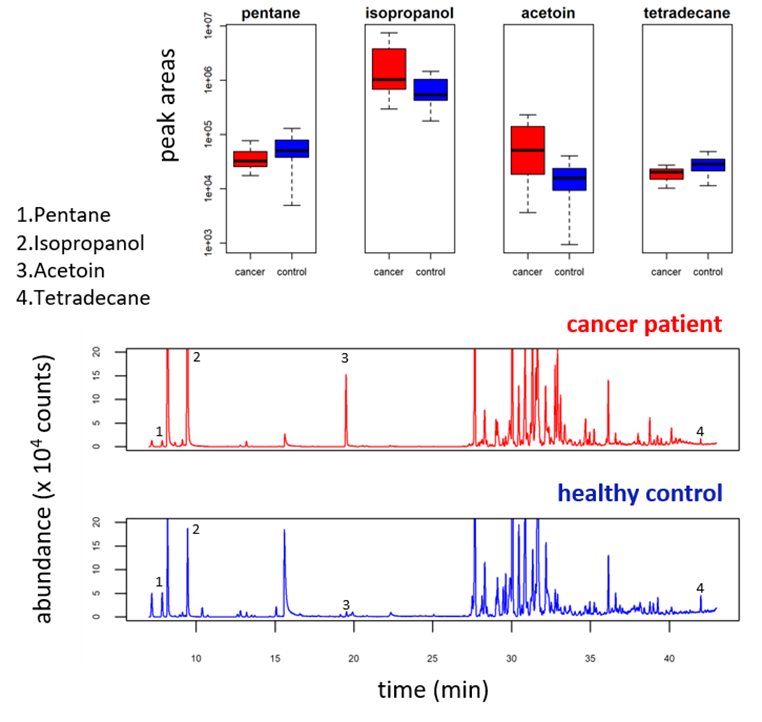
Figure 1. VOC profiles from the breath of a patient and a healthy control, as obtained by TD–GC–MS. Compounds 1-4 are consistently up / downregulated in the breath of cancer patients. (Markar, S. R., Brodie, B., Chin, S.-T., Romano, A., Spalding, D., & Hanna, G. B. (2018). Profile of exhaled-breath volatile organic compounds to diagnose pancreatic cancer. British Journal of Surgery, 105(11), 1493–1500. https://doi.org/10.1002/bjs.10909).
Historically, breath research has been mostly done in academic research labs, but in recent years several companies have emerged onto the commercial breath research scene. One of the largest players in this field is Cambridge-based Owlstone Medical with their Breath Biopsy® product in use in more than 100 clinics worldwide.
This development of this field of study offers the intriguing possibility of breath analysis making the leap from research to clinical practice. This would open up the screening of large populations, improving patient survival and reducing costs for health services.
However, a successful transition from benchtop to bedside requires a valid basis for reproducible and standardized breath VOC collection. The resolution of logistical issues such as the collection of breath from a high number of patients across multiple remote locations is one of the challenges.
Approaches to breath sampling
With growing interest in breath monitoring for disease diagnosis researchers have a choice of products and methodologies for sampling. Ideally a breath sampling device should be low cost, portable and easy to use. Storage and transportation of the samples for analysis must also be simple, secure and convenient – thermal desorption tubes provide this. Available options vary in complexity, cost and ability to select specific fractions of exhaled breath, since the composition of exhaled air will progressively change when it moves throughout the airways, from the alveoli to the mouth.
Sampling approaches include:
- Disposable bags made of various materials (e.g. Nalophan, Tedlar, inertised aluminium) can be used to collect a defined volume of breath from a few hundred milliliters to a few liters. However, samples collected in this way have limited stability (from a few hours to a few days, depending on the compound of interest) and bags offer poor transportability. Alternatively, breath samples can be immediately transferred from bags to TD tubes using a portable pumping device (e.g. ACTI-VOC or Easy-VOC). When collecting breath using a bag it should be noted that all fractions of breath including mouth air are sampled without discrimination.
- Markes’ BioVOC-2 breath sampler, a recent introduction to the market – a lightweight and easy-to-use device for non-invasive biological exposure monitoring of VOCs. Using a design pioneered by the UK Health & Safety Executive over two decades ago, BioVOC-2 captures expiratory breath for immediate transfer to sorbent TD tubes. These are then typically analysed via TD–GC–MS. In contrast to use of disposable bags, BioVOC-2 captures just the last 129 mL of expired air, thus reducing contamination with VOCs from the mouth. Whilst the sampler’s predecessor, Bio-VOC has been successfully used for occupational health monitoring and exposure of the public to VOCs, its main area of application is clinical research. Scientists from research groups worldwide have used Bio-VOC in their research and this type of breath sampling is increasingly seen as suitable for large-scale screening of susceptible populations.
- Breath sampling devices of varying degrees of sophistication have been developed by research groups worldwide. The ReCIVA® from Owlstone Medical represents a commercial solution which allows simultaneous, direct transfer of a fixed volume of exhaled breath onto four TD tubes using a disposable face mask and built-in pumps. Pressure and CO2 sensors allow specific fractions of exhaled breath to be selected ensuring reliable, reproducible collection of VOCs. Subjects breathe a controlled supply of air, and samples of their exhaled breath are captured and stabilised on Breath Biopsy Cartridges, which can then be shipped for analysis with Owlstone Medical’s Breath Biopsy analytical platform to determine their VOC profile. Advanced data analytics techniques can then be applied in order to pinpoint the VOCs of interest. Owlstone is currently running a number of clinical studies including the world's largest breath based clinical trial.
Breath and beyond
The prevalence of studies dealing with VOC analysis within different types of biological matrices has been increasing in recent years. The most represented biological matrix is breath, but bodily fluids (e.g. blood or urine) or tissues are also studied alongside breath to confirm the identity of biomarkers and establish their kinetics of distribution throughout the body. The increase in scientific production in the field of VOC analysis is also accompanied by an increasing interest from the clinical community, as witnessed by the recent rise in the number of clinical trials involving breath analysis.





